Chapter Overview:
Everything in this universe is made up of material which scientists call "matter". This chapter deals
with the physical nature of matter, its particles, states of matter (Solid, Liquid, Gas), and their
inter-conversion through Temperature and Pressure effects, and the phenomenon of Evaporation.
1. Physical Nature of Matter
Definition: Anything that occupies space (Volume) and has Mass is called Matter. E.g.,
Air, Food, Stones, Clouds, Stars.
Important SI Units:
- Mass: Kilogram ($kg$)
- Volume: Cubic metre ($m^3$). (Common unit: Litre ($L$)). $1 L = 1 dm^3$, $1 L =
1000 mL$, $1 mL = 1 cm^3$.
- Density: Mass per unit volume. Density = $\frac{Mass}{Volume}$. SI Unit: $kg/m^3$.
- Pressure: Pascal ($Pa$). Atmosphere ($atm$) is a common unit. $1 atm = 1.01 \times
10^5 Pa$.
Characteristics of Particles of Matter
- Made of tiny particles: Matter is particulate, not continuous (like a block of wood).
Dissolving KMnO₄ crystals in water shows that just a few crystals can color a large volume of water
(Millions of particles in one crystal).
- Have space between them: When we make tea, coffee, or lemonade, particles of one type
of matter get into the spaces between particles of the other.
- Continuously moving: Particles possess Kinetic Energy. As temperature rises, particles
move faster.
Example: Smell of hot sizzling food reaches you several meters away, but for cold food, you
have to go close (Diffusion).
- Attract each other: Force of attraction keeps particles together. Strength of force
varies (e.g., easy to break a stream of water with hand, but impossible to break an iron nail).
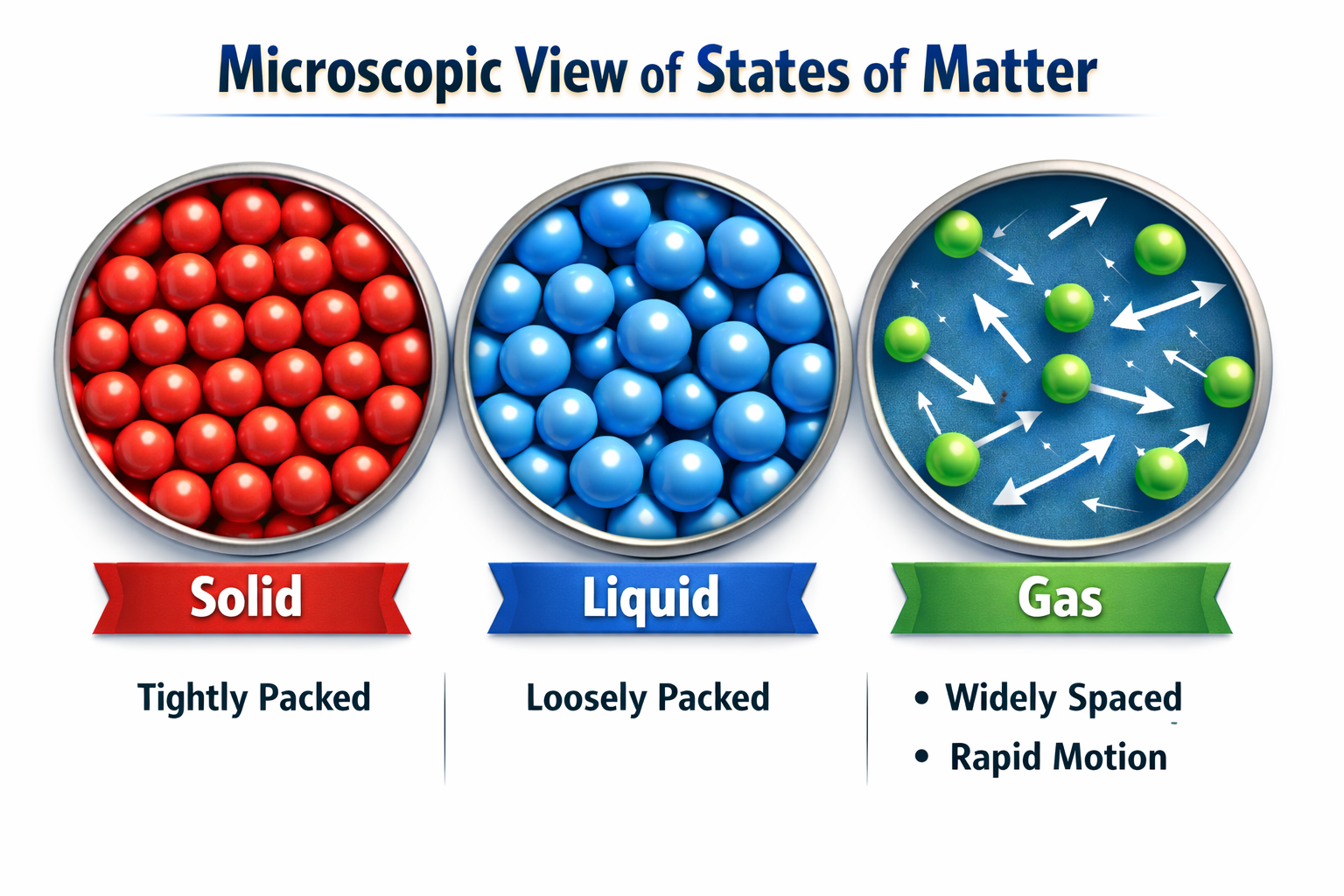
2. States of Matter
Matter around us exists in three states based on particle arrangement.
| Property |
Solid |
Liquid |
Gas |
| Shape & Volume |
Definite shape, Fixed volume. |
No fixed shape (takes shape of container), Fixed volume. |
No fixed shape, No fixed volume. |
| Compressibility |
Negligible. |
Low. |
High (e.g., LPG, CNG cylinders). |
| Particle Packing |
Very close (Ordered). |
Less close (Can slide over each other). |
Free to move (Far apart). |
| Force of Attraction |
Maximum. |
Medium. |
Minimum. |
| Kinetic Energy |
Minimum (Vibrate at position). |
More than solids. |
Maximum (Move randomly). |
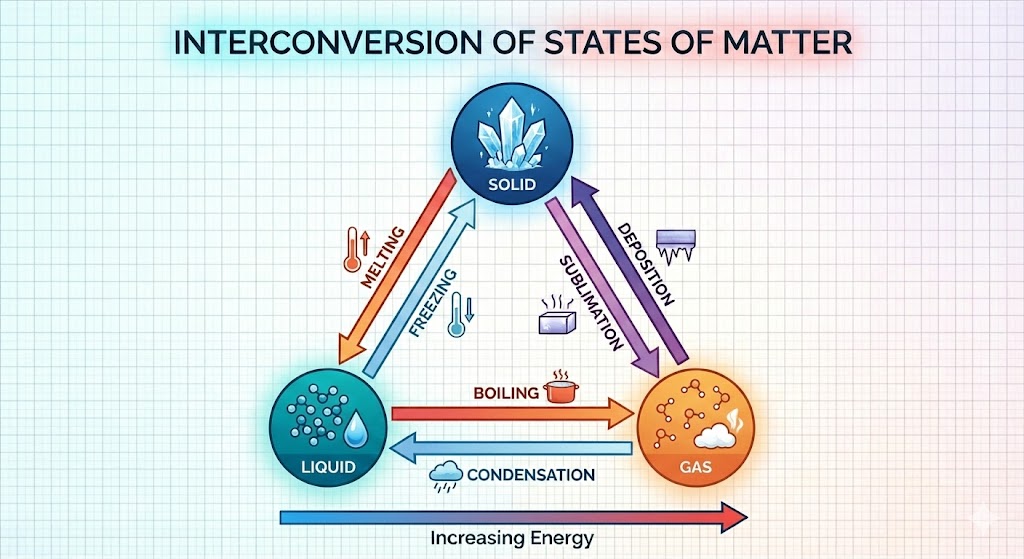
3. Change of State of Matter
Matter can change from one state to another by changing Temperature or Pressure.
(a) Effect of Change of Temperature
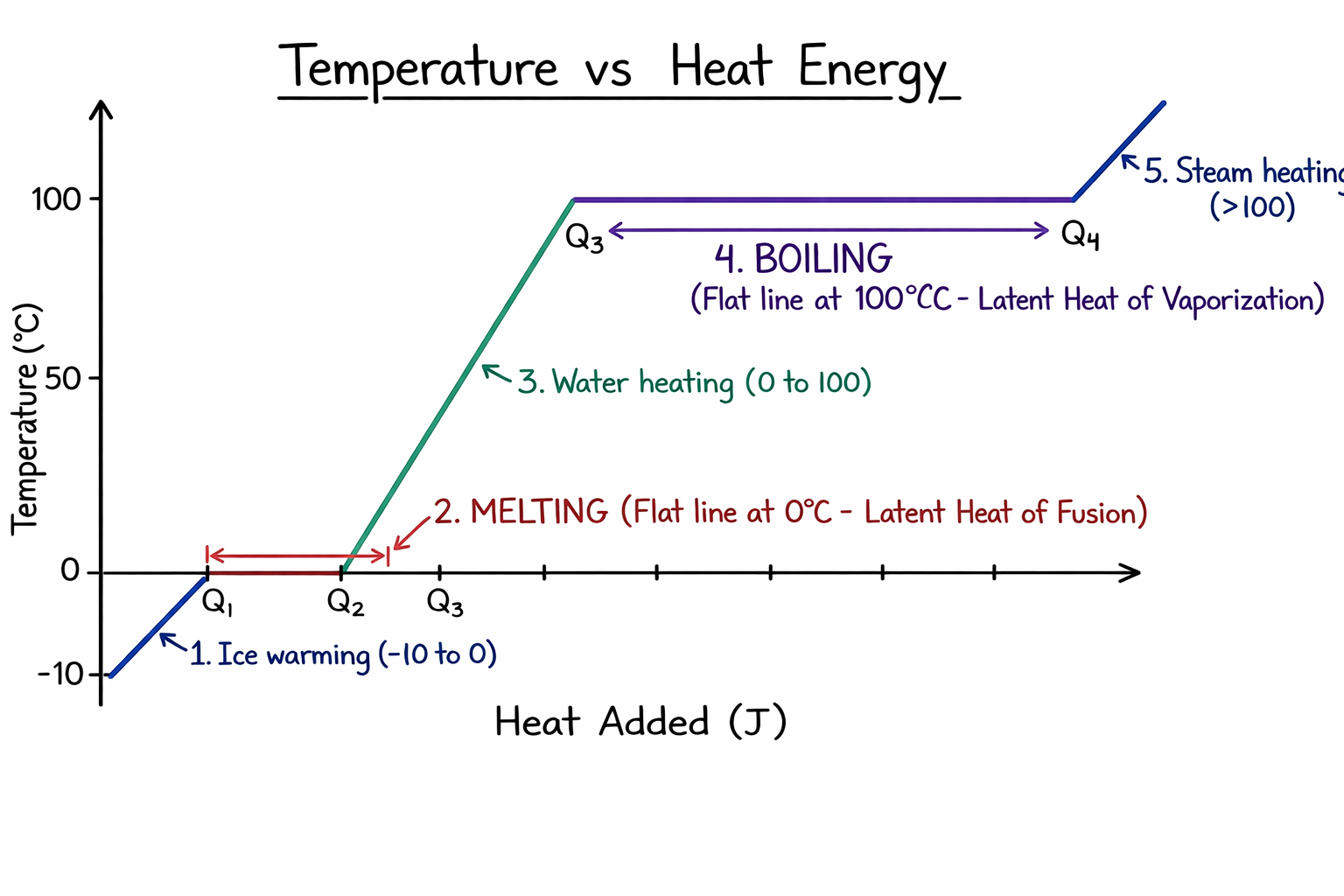
- Melting Point (Solid to Liquid): The temperature at which a solid melts to become a
liquid at atmospheric pressure.
Ice melts at $0^\circ C$ (273.15 K).
- Boiling Point (Liquid to Gas): The temperature at which a liquid starts boiling at
atmospheric pressure.
Water boils at $100^\circ C$ (373 K).
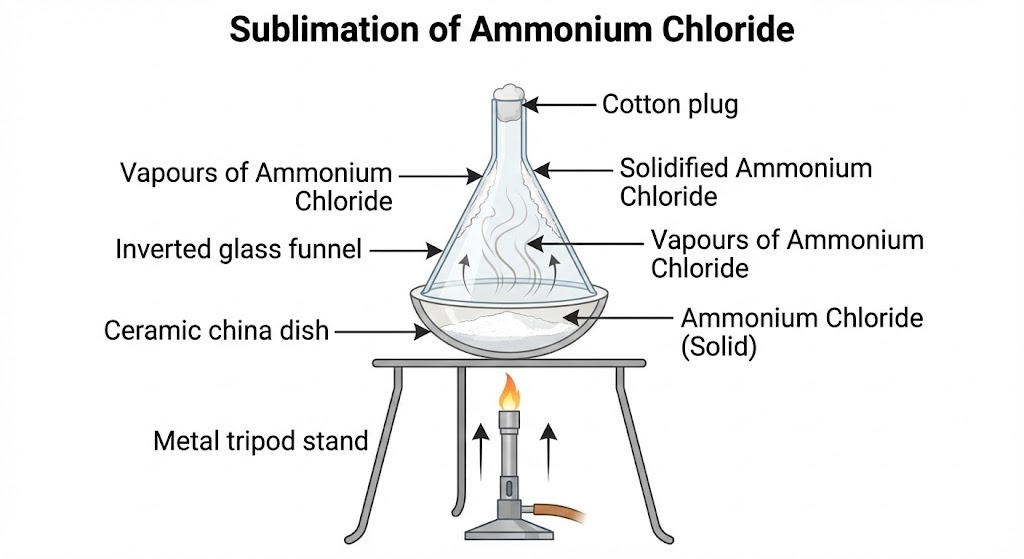
- Sublimation: Change of state directly from solid to gas without changing into
liquid state (e.g., Camphor, Ammonium Chloride).
- Deposition: Direct change of gas to solid without changing into liquid.
(b) Effect of Change of Pressure
Applying pressure and reducing temperature can liquefy gases.
Solid Carbon Dioxide ($CO_2$): Also known as Dry Ice. It is stored
under high pressure. If pressure decreases to 1 atmosphere, it converts directly to gas (Sublimation)
without becoming liquid. This is why it's called 'dry' ice.
4. Temperature Conversion
5. Evaporation
Definition: The phenomenon of change of a liquid into vapours at any temperature
below its boiling point is called evaporation. Unlike boiling (a bulk phenomenon),
evaporation is a surface phenomenon.
Factors affecting Evaporation:
- Surface Area: Increased surface area increases evaporation (e.g., Spreading out wet
clothes to dry).
- Temperature: Higher temp $\rightarrow$ higher kinetic energy $\rightarrow$ faster
evaporation.
- Humidity: Amount of water vapour in air. Higher humidity $\rightarrow$ lower
evaporation.
- Wind Speed: Wind moves water vapour away $\rightarrow$ faster evaporation.
Evaporation causes Cooling:
Particles of liquid absorb energy from the surroundings to regain the energy lost during evaporation,
making the surroundings cool.
- Acetone/Perfume: When poured on palm, particles gain energy from palm/surroundings
and evaporate, causing cooling sensation.
- Cotton Clothes in Summer: Cotton is a good absorber of water. It absorbs sweat and
exposes it to the atmosphere for easy evaporation, cooling the body.
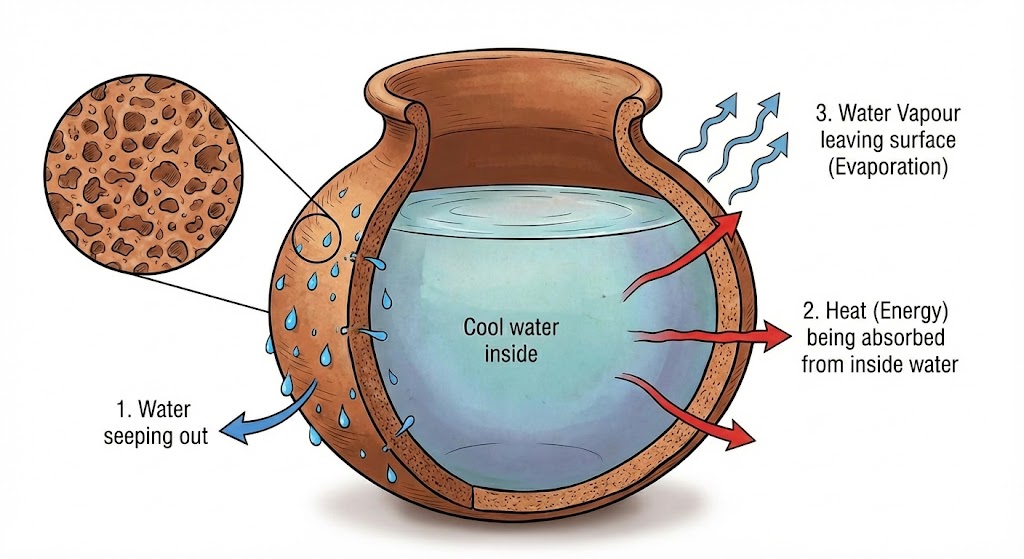
- Earthen Pot (Matka): Water seeps through tiny pores, evaporates from the surface,
and takes latent heat from the remaining water, cooling it down.
6. Two More States of Matter (Bonus)
- Plasma: Super energetic and super excited particles. These particles are in the form of
ionized gases.
Example: Fluorescent tube (helium gas), Neon sign bulbs (neon gas). The sun and stars glow
because of plasma created by high temperature.
- Bose-Einstein Condensate (BEC): Formed by cooling a gas of extremely low density (one
hundred-thousandth of normal air) to super-low temperatures. Predicted by Albert Einstein and Satyendra
Nath Bose.
Q1: Convert 573 K to Celsius scale.
Ans: $573 - 273 = 300^\circ C$.
Q2: Why is ice at 273 K more effective in cooling than water at the same temperature?
Ans: Ice at 273 K ($0^\circ C$) has less energy than water at the same temperature
because water particles have absorbed extra energy in the form of latent heat of fusion
during melting.